產品分類
快速訂購
31952518
- 029-86354885
- 18392009562
sales@xarxbio.com
DBCO-PEG12-MMAE
| Catalog # | Pkg Size | Price | Quantity | Shopping Cart |
|---|---|---|---|---|
| R-CD-039 | 1mg | ¥ 2384 | 加入購物車 | |
| R-CD-039 | 5mg | ¥ 4720 | 加入購物車 | |
結構式
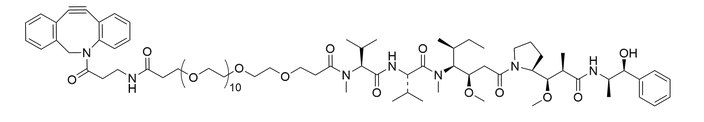
| 外觀 | Slightly yellow to slightly oil |
| 分子量 | 1647.97 |
| 溶解度 | DMSO, DMF, THF, DCM |
| CAS號 | N/A |
| 質量控制 | 95% |
| 儲存條件 | -20°C |
| 保存時間 | 1 year |
| 其他信息 | N/A |
DBCO-containing auristatin drug used to create antibody-drug conjugates via SPAAC reaction. DBCO-PEG12-MMAE contains a long hydrophilic polyethylene glycol (PEG) spacer arm that imparts water solubility and provides a long and flexible connection that minimizes steric hindrance involved with ligation to complementary azide-containing molecules.
親,說點什么吧,您的評價對其它買家很有幫助哦~
“好評,中評,差評”請填寫其一哦~
您的昵稱
相關產品
| DBCO-C6-NHS Ester | DBCO-C6-NHS Ester is an amine-reactive building block used for modification of amine-containing molecule in organic media. |
| DBCO-NHS Ester | DBCO-NHS Ester is an amine-reactive building block used for modification of amine-containing molecule in organic media. |
| DBCO-Sulfo-NHS Ester | DBCO-Sulfo-NHS Ester is water-soluble reagent that enables simple and efficient labeling of antibodies, proteins and any other primary amine-containing macromolecules with DBCO moiety in 100% aqueous buffers. |
| DBCO-PEG4-NHS Ester | DBCO-PEG4-NHS Ester reacts specifically and efficiently with a primary amine (e.g., side chain of lysine residues or aminosilane-coated surfaces) at pH 7-9 to form a covalent bond. |
| DBCO-PEG5-NHS Ester | DBCO-PEG5-NHS Ester reacts specifically and efficiently with a primary amine (e.g., side chain of lysine residues or aminosilane-coated surfaces) at pH 7-9 to form a covalent bond. |
| DBCO-PEG13-NHS Ester | DBCO-PEG13-NHS Ester reacts specifically and efficiently with a primary amine (e.g., side chain of lysine residues or aminosilane-coated surfaces) at pH 7-9 to form a covalent bond. The hydrophilic polyethylene glycol (PEG) spacer arm imparts water solubility and provides a long and flexible connection that minimizes steric hindrance involved with ligation to complementary azide-containing molecules. |


 400-115-0588
400-115-0588 在線咨詢
在線咨詢 Datasheet
Datasheet MSDS
MSDS COA
COA







 庫存查詢
庫存查詢